Dissolved Gases, Air Pockets, and Pressure Dynamics in Domestic Well Water Systems
Homeowners on private wells sometimes encounter dramatic bursts of air or frothy water when they open an outdoor spigot after the line has sat unused. Although it looks like a plumbing defect, the root cause is usually a mix of physics, hydrogeology, and system design. This article explains, in a general and indirect way, how large volumes of air (or gas) can appear in a well supplied water system, why it often shows up after periods of stagnation, and what processes inside pipes, tanks, and aquifers make it happen. The discussion is framed to be broadly useful rather than focused on any single case.
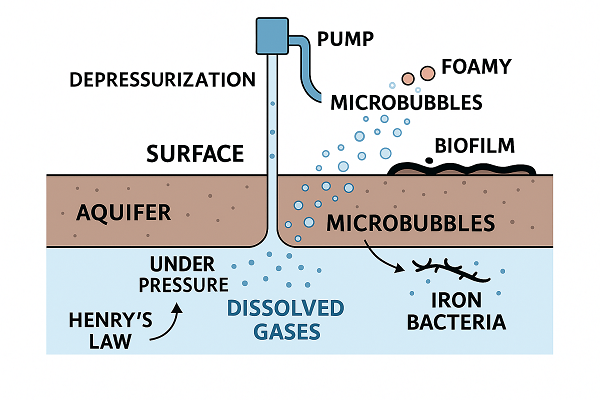
Groundwater as a Gas Reservoir
Groundwater is rarely pure H₂O. It carries dissolved minerals (iron, manganese, calcium) and dissolved gases (nitrogen, oxygen, carbon dioxide, methane, hydrogen sulfide). The amount of gas that water can hold depends on pressure, temperature, and the specific gas species. According to Henry’s law, gas solubility increases with pressure and decreases with temperature. Deep inside a confined aquifer, pressures are higher than at the surface; water can therefore carry more dissolved gas. When that water is pumped to the surface and depressurized, the solution becomes supersaturated and the gas comes out as microbubbles. Think of opening a carbonated drink: depressurization drives rapid degassing.
Iron-rich wells often amplify the visual effect. Iron oxidizes when it meets oxygen, creating reddish precipitates and sometimes ferric floc that traps bubbles, making the discharge seem especially “foamy.” If iron bacteria are present, their metabolic activity can also generate gases and slimy biofilms that trap bubbles along pipe walls.
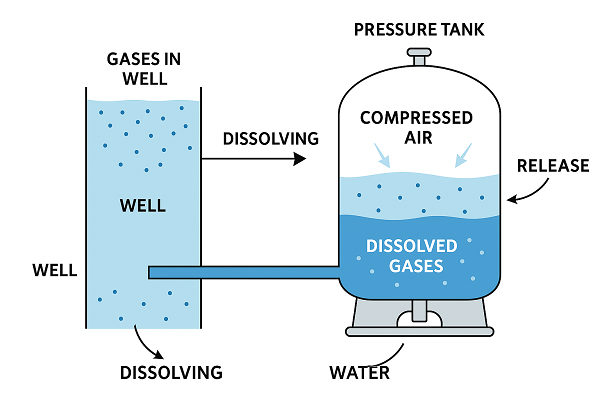
Pressure Tanks, Air Cushions, and Bladders
Most residential well systems use a pressure tank to buffer pump cycling. Two broad designs exist:
-
Older galvanized steel tanks without bladders. These rely on an air cushion above the water to provide compressibility. Over time, air dissolves into the water and must be replenished with air chargers or snifters. An imbalance can lead to either waterlogging (no air) or excess air being swept into the distribution lines.
-
Modern diaphragm or bladder tanks. A rubber membrane separates compressed air on one side from water on the other. In theory, no air should enter the plumbing. However, if the bladder fails, or if there are ancillary components (snifter valves, air volume controls) still in play, air can migrate.
Even with a perfect bladder tank, pressure fluctuations matter. When the pump stops at the high setpoint and a long branch line remains static, temperature shifts and minor leaks can lower pressure locally, letting dissolved gas come out of solution inside that branch. When the line is finally opened, the accumulated gas rushes out first, followed by water.
Long, Lightly Used Exterior Runs
Outdoor hose bibbs are often fed by long runs of small-diameter PEX or PVC. Several characteristics of these runs favor gas accumulation:
-
Low flow frequency: Rare use means the water column sits undisturbed for days, letting gases nucleate on pipe walls and coalesce into slugs.
-
Thermal swings: Sun-exposed or shallow-buried lines heat and cool daily, changing gas solubility. Warmer water releases more gas.
-
High points: Any vertical rise creates traps where buoyant bubbles collect. Unlike interior plumbing that may self-bleed through frequent use, outdoor runs become de facto air separators.
These factors explain why an outside tap, after a week of inactivity, may spew 20 minutes of spitting air even though indoor fixtures never show the problem.
No-Leak Systems Still Breathe
Homeowners often conclude that there must be a suction-side leak pulling air in. While that is one possibility, especially if the foot valve or drop pipe has issues, a perfectly sealed system can still produce apparent “air” through degassing. Water does not need to leak for gas to appear; it only needs to experience a pressure drop or temperature rise after it left the pressure tank. Moreover, tiny microbubbles can pass check valves without creating obvious backflow symptoms.
Distinguishing Air from Combustible Gas
Bubbles at a faucet can be air (mostly nitrogen/oxygen), carbon dioxide, methane, or hydrogen sulfide. Trying to ignite bubbles is a crude but common field test. Methane will burn with a blue flame, but ignition is unreliable in a wet stream. Laboratory gas chromatography or handheld meters provide better identification. For domestic risk assessment:
-
Rotten egg smell indicates hydrogen sulfide (nonflammable at low concentrations but corrosive and odorous).
-
Metallic taste and orange staining suggest iron oxidation, not gas toxicity.
-
Headaches or dizziness in enclosed pump rooms could indicate CO₂ accumulation.
If there is any suspicion of methane, a well professional or local water lab should test the raw water. Methane is not dangerous to drink but can pose explosion hazards if it accumulates in confined spaces.
How Stagnation Amplifies Gas Release
When water sits motionless in a pipe, three processes encourage gas pockets:
-
Nucleation on surfaces: Tiny defects and biofilms act as sites where dissolved gas molecules cluster and form bubbles.
-
Coalescence and buoyancy sorting: Over hours or days, microbubbles merge into larger pockets that float to the highest points in the run.
-
Diffusion gradients: Gas can slowly diffuse from water into the pocket, enlarging it even without significant pressure change.
When the valve opens, the first slug ejected is mostly gas, followed by aerated water, then normal flow. If the line is extremely long, multiple slugs may form, leading to extended spitting.
Interaction with Treatment Equipment
Systems that include iron filters, water softeners, and tannin removers introduce additional head loss and pressure fluctuations. If outdoor lines are plumbed upstream of those devices, they may experience different pressures and flow patterns than the indoor plumbing. Bypass valves or diversions may create zones where water stagnates longer. Furthermore, media tanks can trap gas and burp it intermittently after regeneration cycles.
Diagnosing Without Tearing Everything Apart
A structured approach helps:
-
Compare fixtures: Do indoor faucets show microbubbles that clear after a second? If yes, degassing is systemic. If only remote lines sputter, suspect stagnation effects.
-
Time correlation: Note how many days of inactivity are needed before large air events occur. Longer idle periods with worse symptoms point to in-line gas growth rather than a suction leak.
-
Pressure logging: A simple gauge or electronic logger at the far end can reveal pressure drops overnight. Even a few psi drop can trigger bubble formation.
-
Sample raw well water: Draw water at the wellhead before any tank or filter. Observe if bubbles form in a sealed jar over several hours. Testing can quantify methane, CO₂, and H₂S.
-
Inspect tank internals: Verify bladder integrity (tap test, weight, or removal if necessary). Make sure no air-charging devices are active unless required.
-
Check for snifter/check valve combinations: Some systems intentionally inject air into the tank to maintain an air cushion. If retrofitted with a bladder tank, these legacy parts can cause chronic air discharge.
Mitigation Strategies
Depending on findings, several remedies exist:
-
Bleed valves at high points: Manual or automatic air vents on long exterior runs let gas escape before it accumulates.
-
Looped piping or recirculation: Designing the network so that water circulates periodically (for example, with a timed flush) prevents long stagnation.
-
Relocate takeoffs: Feed outdoor taps after treatment equipment and closer to the pressure tank to reduce stagnation length and pressure swings.
-
Install an inline microbubble separator: Devices used in hydronic heating can also strip dissolved gases from water in plumbing loops.
-
Temperature control: Bury exterior lines deeper or insulate exposed sections to minimize daily temperature swings that drive degassing.
-
Address source gas: If methane or hydrogen sulfide levels are high, aeration tanks, stripping towers, or catalytic carbon filters can remove them before the water enters the household plumbing.
-
Maintain iron filters and softeners: Proper backwashing and regeneration keep media from fouling and reduce zones where gas can accumulate.
The Role of Iron and Biofilms
High iron content, especially combined with iron bacteria, creates two noticeable phenomena: orange staining and copious tiny bubbles. Iron bacteria oxidize ferrous iron to ferric iron, forming gelatinous deposits that adhere to pipe walls. These deposits trap microbubbles more effectively than smooth plastic tubing. When flow resumes, chunks of biofilm and gas pockets break free together, producing a burst of foam. Shock chlorination of the well and lines can reduce bacterial loads, but frequent reintroduction is common if the aquifer itself hosts the bacteria.
Why You Cannot Always “Flush It Out” Quickly
Owners often report that it takes 20–30 minutes to clear the air. This duration is a clue to system volume and gas generation rate. If a 50 ft run of 3/4 inch PEX contains roughly 1.2 gallons of water, a 30 minute purge at typical outdoor flow rates suggests sequential release of multiple pockets rather than a single trapped slug. Each pressure drop as a pocket moves can nucleate more bubbles downstream, prolonging the event. Moreover, as flow begins, turbulence scrubs more dissolved gas out of the water, extending the bubbly phase.
Preventive Design Lessons
New installations can minimize future air issues by following a few design principles:
-
Keep branch lines short and avoid unnecessary high points.
-
Provide a common manifold close to the pressure tank, with individual, valved stubs to outdoor fixtures so they can be isolated and drained seasonally.
-
Use sloped piping and strategically placed vents where unavoidable rises occur.
-
Separate treatment loops from raw water bypasses to eliminate long, stagnant segments.
Conclusion
What looks like mysterious air intrusion in a well-based plumbing system is often the predictable outcome of dissolved gas behavior under changing pressures and temperatures, compounded by stagnation in long exterior lines. Understanding the interplay between the aquifer, pressure tank dynamics, pipe geometry, and water chemistry allows homeowners and professionals to distinguish harmless degassing from genuine mechanical faults. With proper diagnosis and targeted adjustments—whether venting a high point, relocating a tap, or removing methane at the source—the annoyance of prolonged sputtering can usually be reduced or eliminated.
The next time a hose spits and hisses for half an hour, remember: your plumbing is acting like a miniature gas separator. The solution may not be to hunt for leaks, but to manage where and how the gas leaves the water before it reaches your faucet.
